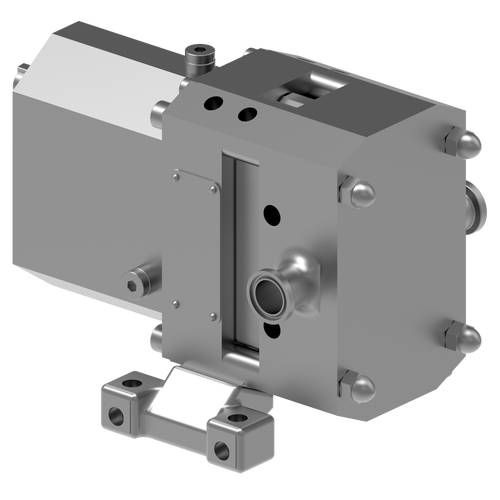
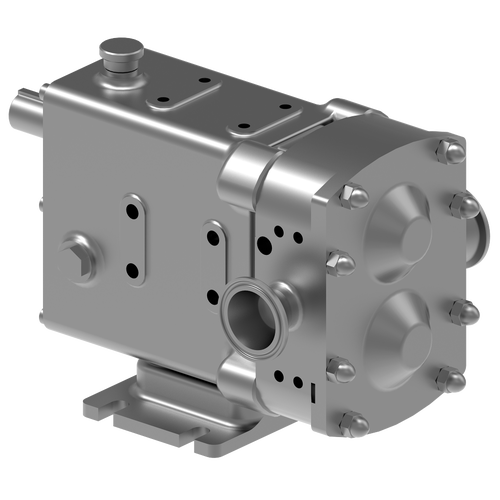
30+ Years of Proven Solutions in Biopharma Fluid Conveyance & Pharmaceutical Pumps
Viking Pump® Hygienic develops and manufactures rotary lobe pumps for critical applications in multiple-use biopharmaceutical and pharmaceutical applications. Our rotary lobe pump technology is designed to be specifically gentle on proteins and cells in process fluids, inherently clean with clean-in-place (CIP systems) and sterilize-in-place (SIP) capabilities, and simple to validate thanks to our complete range of certifications and documentation packages. Furthermore, our complete range of products empowers your customers with a direct path to scalability from R&D to mass production environments.
You are faced with challenges every day
- | Inherent drainability shortcomings common in rotary lobe pumps
- | Skid size restrictions make orienting pumps vertically for best drainage difficult
- | Specific and various piping requirements depending on the customer's specifications
- | Long lead times and increased cost when sourcing the configuration you need
Viking Pump Hygienic is here to help
- | We offer the lowest dead-leg possible for a horizontal ported rotary lobe pump. This maximizes your product recovery
- | Optimized footprint with quick assembly and readily available gaskets and fasteners
- | Connect your drainpipe directly to the front cover for ultimate flexibility
- | One part number for any application for faster delivery and hassle-free processing

Role of Rotary Lobe Pumps in Biomanufacturing
Pumps play a crucial role in biomanufacturing processes, transferring materials such as media, buffers, and final products. Among the options available, Viking Pump Hygienic's rotary lobe pump stands out as the perfect solution. The hygienic design, gentle fluid handling, and dynamic control when it comes to flow rates and fluid flows offer superior results to the final product.
Get In Touch With The Experts

Strategic Key Account Manager - Biopharma
The SteriLobe® Series
1 | IMPROVED YIELD
Rotary lobe pumps offer high efficiency pumping at full range. The flow rate is largely independent of changes in pressure. Additionally, you can expect low shearing and expert handling of fluids prone to flashing.
The flow through a rotary lobe pump is proportional to speed. It provides easy control of flow rate with a variable speed drive for excellent metering capabilities. Paired with simple maintenance that can be done without removing the pump from the system, the SteriLobe® rotary lobe pump is your best solution for your pharmaceutical application.
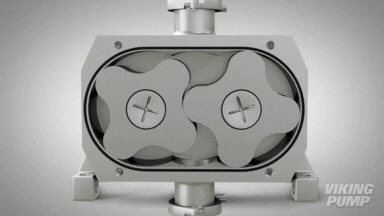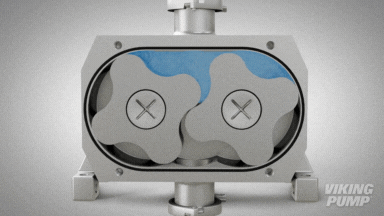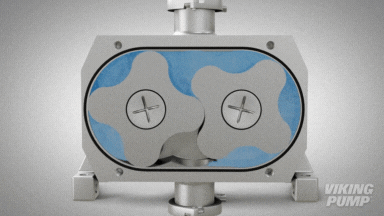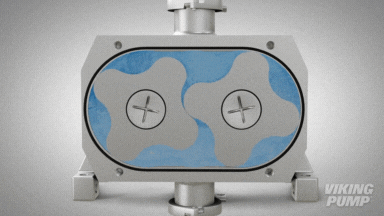
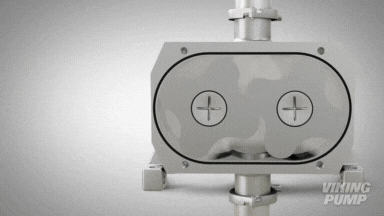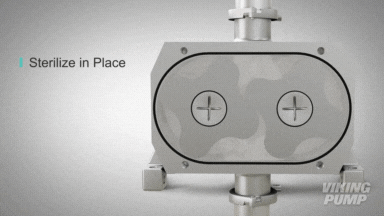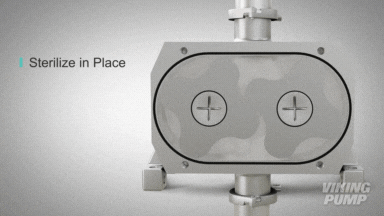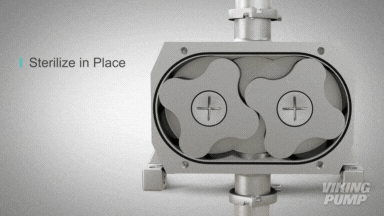
2 | CLEAN IN PLACE (CIP) & STERILIZE IN PLACE (SIP)
With an aseptic design the SteriLobe® can undergo optimum cleaning in place (CIP) to meet strict cleaning parameters. If your system has a high temperature design, the SteriLobe® can also sterilize in place (SIP) with steam (compared to manual cleaning which takes much longer and is less efficient).
3 | SCALABILITY
Product offerings from benchtop system with flow as low as 10 l/min up, through pilot scale flow range up to full production with flow up to 2 m³/min (120,000 l/hour).
The Efficient Cleaning Process
Equipment cleaning requires a set cleaning regime for clean in place or sterilize in place process equipment- and the SteriLobe® Series is designed to be integrated with these effective cleaning methods. A fully stainless-steel design with options for enhanced surface finish and electropolishing to all media contact surfaces allows for complete sterilization without removal from the process. Vertical porting and relieved cusp features in the casing allow for the pump to completely self-drain process liquid prior to the cleaning cycle and for the complete draining of chemical solutions after the cleaning is complete.
Related Liquids
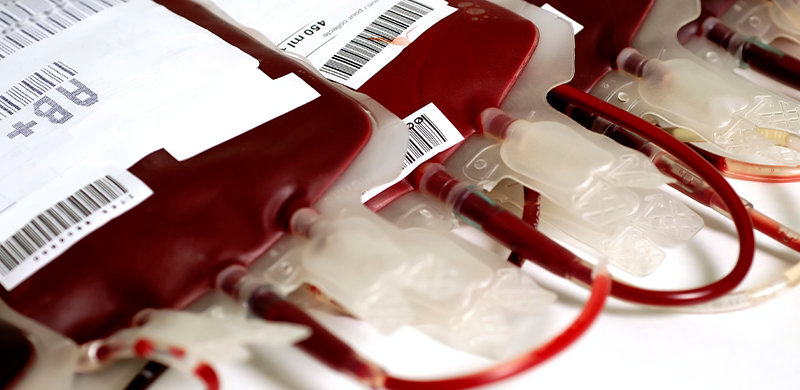
Produits sanguins
Produits thérapeutiques dérivés du sang humain, y compris le sang total, le plasma et d'autres composants sanguins destinés à la transfusion et au traitement médical.
Bouillon de cellules
Les cellules, les nutriments et les autres composants qui constituent le contenu d'un bioréacteur microbien. Ils sont souvent utilisés dans les produits biopharmaceutiques.

Vaccin

Solution protéique

3-A Sanitary Standards, Inc. (3-A SSI) is an independent, not-for-profit corporation dedicated to advancing hygienic equipment design for the food, beverage, and pharmaceutical industries. 3-A SSI represents the interests of these three stakeholder groups with a common commitment to promoting food safety and the public health regulatory sanitarians, equipment fabricators and processors. Viking Pump Hygienic has achieved 3-A certifications across our product lines and is actively involved in 3-A standards development.

The CE marking symbolizes the conformity of Viking Pump Hygienic’s products with the applicable European Union (EU) legislative requirements as stated in the Machinery Directive 2006/42/EC. The CE marking affixed to Viking Pump Hygienic’s products is a declaration of our products conformance to all applicable EU provisions and the appropriate conformity assessment procedures successfully completed.

The ATEX (EX) marking symbolizes the conformity of Viking Pump Hygienic’s products with the applicable European Union (EU) legislative requirements in addition to those required by Machinery Directive 2006/42/EC for CE marking. The ATEX Directive applies to pumps intended for use in potentially explosive atmospheres. The EX marking affixed to Viking Pump Hygienic’s products is a declaration of our products conformance to all applicable EU provisions and the appropriate conformity assessment procedures successfully completed.